
Basket designs are prospective clinical trials that are devised with
the hypothesis that the presence of selected molecular features
determine a patient’s subsequent response to a particular “targeted”
treatment strategy. Basket trials are designed to enroll multiple
clinical subpopulations to which it is assumed that the therapy in
question offers beneficial efficacy in the presence of the targeted
molecular profile. The treatment, however, may not offer acceptable
efficacy to all subpopulations enrolled. Moreover, for rare disease
settings, such as oncology wherein these trials have become popular,
marginal measures of statistical evidence are difficult to interpret for
sparsely enrolled subpopulations. Consequently, basket trials pose
challenges to the traditional paradigm for trial design, which assumes
inter-patient exchangeability. The R-package basket
facilitates the analysis of basket trials by implementing multi-source
exchangeability models. By evaluating all possible pairwise
exchangeability relationships, this hierarchical modeling framework
facilitates Bayesian posterior shrinkage among a collection of discrete
and pre-specified subpopulations.
You can install the released version of basket from CRAN with:
install.packages("basket")And the development version from GitHub with:
# install.packages("devtools")
devtools::install_github("kaneplusplus/basket")The “Vemurafenib
in multiple nonmelanoma cancers with BRAF V600 mutations” study
enrolled patients into predetermined baskets that were determined by
organ site with primary end point defined by Response Evaluation
Criteria in Solid Tumors (RECIST), version 1.1 or the criteria of the
International Myeloma Working Group (IMWG). Statistical evidence for
preliminary clinical efficacy was obtained through estimation of the
organ-specific objective response rates at 8 weeks following the
initiation of treatment. This section demonstrates the implementation of
through analysis of six organs comprising non–small-cell lung cancer
(NSCLC), cholangiocarcinoma (Bile Duct), Erdheim–Chester disease or
Langerhans’-cell histiocytosis (ECD or LCH), anaplastic thyroid cancer
(ATC), and colorectal cancer (CRC) which formed two cohorts. Patients
with CRC were initially administered vemurafenib. The study was later
amended to evaluate vemurafenib in combination with cetuximab for CRC
which comprised a new basket. Observed outcomes are summarized below.
Included in the package, the dataset is accessible in short
vemu_wide as well as long formats vemu.
library(basket)
data(vemu_wide)
vemu_wide
#> # A tibble: 6 × 7
#> baskets enrolled evaluable responders one_or_fewer_pr… two_prior_thera…
#> <chr> <dbl> <dbl> <dbl> <dbl> <dbl>
#> 1 NSCLC 20 19 8 11 4
#> 2 CRC (vemu) 10 10 0 1 2
#> 3 CRC (vemu+cetu) 27 26 1 5 11
#> 4 Bile Duct 8 8 1 2 1
#> 5 ECD or LCH 18 14 6 9 7
#> 6 ATC 7 7 2 5 1
#> # … with 1 more variable: three_or_more_therapies <dbl>Inspection of Table reveals heterogeneity among the studied baskets.
CRC (vemu), CRC (vemu+cetu), and Bile Duct had relatively low response
rates when compared to other baskets, suggesting that patients
presenting the BRAF V600 mutation may not yield exchangeable information
for statistical characterization of the effectiveness of the targeted
therapy. Therefore, the MEM framework is implemented to measure the
extent of basketwise heterogeneity and evaluate the effectiveness of the
targeted therapy on the basis of its resultant multi-resolution smoothed
posterior distributions. This case study reports posterior probabilities
evaluating the evidence that the response probability for each
organ-site exceeds the null rate of p0 = 0.25.
An analysis of the trial data can be reproduced by loading the
vemu_wide data, which is included with the package. The
data set includes the number of evaluable patients (column
evaluable), the number of responding patients (column
responders), and the associated baskets for the respective
results (column baskets). The model is fit by passing these
values to the basket() function along with an argument
specifying the null response rate of 0.25 for evaluation of each basket.
A visualization of the posterior distribution of the response rates can
be created with the following and shows that the Bile Duct and CRC
cohorts are similar and do not respond to treatment where ATC, ECD or
LCH, and NSCLC do respond.
data(vemu_wide)
vm <- basket(vemu_wide$responders, vemu_wide$evaluable,
vemu_wide$baskets, p0 = 0.25)
plot_density(vm, type = "basket")
#> Warning: It is deprecated to specify `guide = FALSE` to remove a guide. Please
#> use `guide = "none"` instead.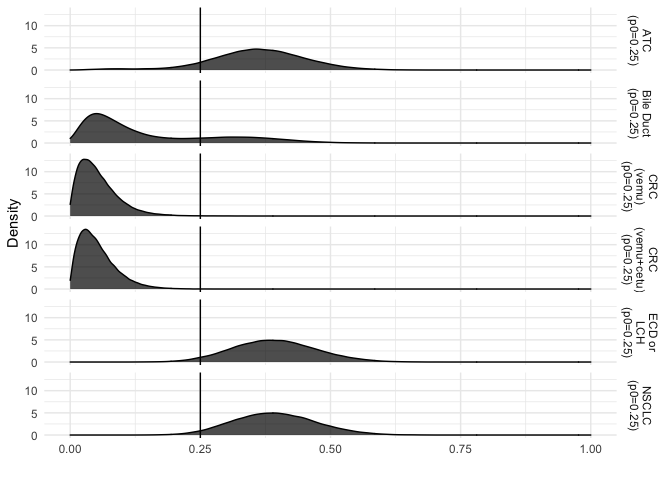
Please note that this project is released with a Contributor Code of Conduct. By participating in this project you agree to abide by its terms.